Water Phase Diagram
4 stars based on
33 reviews
Vaporization is the process of converting a liquid into a gas. It is also called evaporation. Since we know that the particles of a gas are moving faster than those of a liquid, an input of energy must be required for a liquid to become a gas.
The most common way to add energy to a liquid system is by adding heat. As a liquid gains energy, the molecules begin to move can both liquid water and water vapor exist at 100 degrees celsius faster.
If a molecule is on the surface of the liquid, and has enough energy, it can break free and become a gas molecule. As with anything in chemistry, or life for that matter, there are other factors that determine how easily a molecule can break free from the liquid. We just discussed some of them: The stronger the intermolecular forces that are holding a liquid together, the more energy that will be required to pull them apart.
What this means in practicle terms is that a liquid with strong intermolecular forces will have to be heated to a higher temperature before it will evaporate. Look at Methane CH 4 M. Their molecular weights are very similar, but their Heats of Vaporization how much heat per mole that has to be added to make them evaporate are very different.
Water has a D H vap of Methane is actually a gas at room temperature because of its low heat of vaporization. Well, the opposite of vaporization, of course. The process by which a gas changes phases into a liqud. What else makes sense is that the amount of energy required to go from gas to liquid phase would be the same as that required to go from liquid to gas, just opposite in sign. Another property that affects the value of the D H vap is the molecular weight or size of the molecule.
This is generally because you have more surface London Force interactions between larger molecules than small and the density of a larger molecule is generally greater than that of a small molecule. Remember, that to become airborne a molecule must have a lower density than the air molecules around it.
Exceptions in the trend will occur when a small molecule is capable of a stronger intermolecular force, E. HF hydrogen bonds and thus has a higher D H vap. So now that you know all about what a D H vap is, why the heck do we care about it? Well, here is a real life use for this value that is important to us all: For fingernail polishes, if you want the polish to dry faster, you use a solvent that has a lower D H vap. Table 2 lits some of the more common solvents used in fingernail polishes.
Methyl Acetate is the preferred solvent for most. This is because not only is it fast drying, it is also somewhat water soluble. Another simple example of how to use the value is determing how much heat it will take to boil water out of a pot? The D H vap for water is If your pot contains 2. The molecular weight of water is We can now use the What if we take it one step further? Let's combine some concepts we learned in CHM with the concept we just discussed and solve the following problem: How much heat energy is required to convert 5.
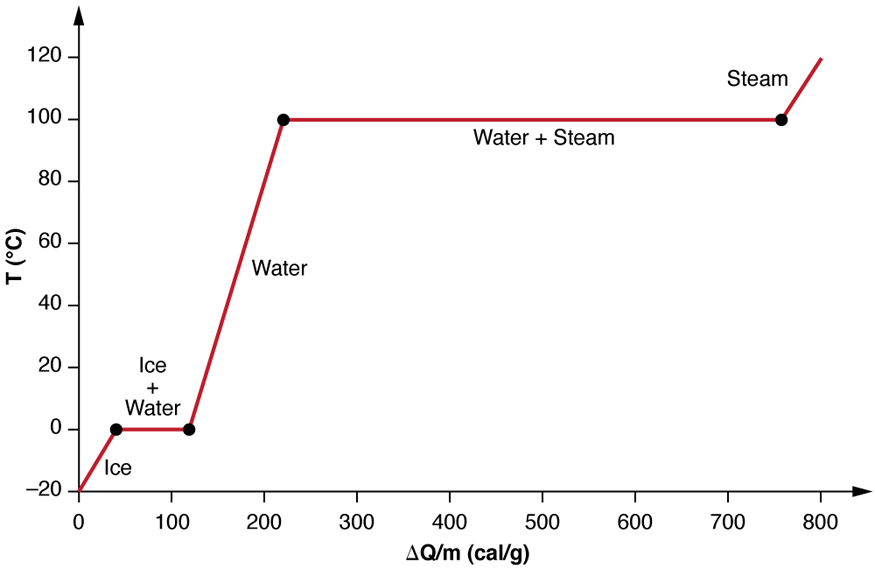
What do we need to calculate? First we need convert the ice from solid to liquid using the Enthalpy can both liquid water and water vapor exist at 100 degrees celsius Fusion a value just like the Heat of Vaporization that measures the amount of heat required to convert a substance from solid to liquid and then we need to heat the material from 0 up to degrees and finally, we need to convert it from liquid to gas using the Heat of Vaporization.
So now we know the three steps to take, we need to collect our conversion factors to use:. So we can simply multiply the number of kg in our. We need to heat the liquid water from 0 to degrees Celsius. The specific heat of water is 4. Convert the liquid water into vapor at o C. For this we use the Heat of Vaporization as we did above.
Vapor pressure is a can both liquid water and water vapor exist at 100 degrees celsius quantity that exists when a liquid and its vapor are in equilibrium. This is only possible in closed systems. But please note that the earth's atmosphere is considered a closed system. On a can both liquid water and water vapor exist at 100 degrees celsius smaller scale this would be when you place any liquid into a sealed container.
The molecules at the surface of the liquid would be changing into gas phase molecules and returning to the liquid until an equilibrium was reached. This is what we call a "dynamic can both liquid water and water vapor exist at 100 degrees celsius. As molecules from the liquid move into the gas phase within the container, this increases the pressure above the liquid. A measure of this pressure minus the normal atmospheric pressure gives us the vapor pressure of the liquid.
The higher this pressure, the more volatile a liquid is said to be. Vapor pressure is also dependent on the temperature of the system.
The liquid will still have a heat of vaporization to contend with so the equilibrium vapor pressure will increase at higher temperatures. And anyone who has ever microwaved their lunch in a closed tuperware container knows what will happen if the pressure or temperature gets too high, don't they? You are can both liquid water and water vapor exist at 100 degrees celsius away for the weekend and you want to make sure you leave enough water in the cage for your bird so it doesn't die of thirst while you are gone.
If your dorm room has an average temperature of 21 o C how many liters of water should you put out to be sure some remains for the entire weekend? If we assume your dorm room has a volume of about 5. A couple of things to note about the calculation above: Vapor pressure is temperature dependent! So for practical use, we would need to know how the vapor pressure changed with temperature that way you can leave your bird at someone else's house, yeah!
Unfortunately, we quickly see that most vapor pressure relationships with temperature are not linear, which makes predictions difficult if not impossible. Never fear, Clausius-Clapeyron is here!
A German and Frenchman walked into a bar Anyway, A German Physicist named Clausius got together with a French physicist named Clapeyron and devised a neat little equation that converts the non-linear relationship between vapor pressure and temperature into a linear one using the pressures and temperatures of a substance at two points.
Basically, you can use this equation for a number of different purposes: The biggest problem students have with this equation is their inability to complete the mathematical manipulations that go along with its solutions.
In order to practice some problems, click here. The boiling point of a liquid is the temperature at which the liquid will convert to a gas. If the atmospheric pressure at this temperature is given as 1 atmosphere or mmHg, then this temperature is called the normal boiling point of the liquid. Boiling points are therefore pressure dependent. The lower the atmospheric pressure, the lower the boiling point and vice versa. This is because the atmospheric pressure is what is pushing against the surface of the liquid and keeping the liquid down.
When the vapor pressure of the liquid is equal to can both liquid water and water vapor exist at 100 degrees celsius greater than the atmospheric pressure, boiling will occur or in other words vapor will form can both liquid water and water vapor exist at 100 degrees celsius the gas phase molecules will escape from the surface of the liquid you see this can both liquid water and water vapor exist at 100 degrees celsius bubbles. The temperature and pressure at which the line between liquid and gas phase blurs is called the critical temperature and critical pressure, respectively.
At very high temperatures and pressures the properties of a substance resemble those of both a liquid and a gas. The critical point represents the highest temperature and pressure at which the substance can exist as a vapour and liquid in equilibrium.
The phenomenon can be easily explained with reference to the phase diagram for pure carbon dioxide Figure below. This figure shows the areas where carbon dioxide exists as a gas, liquid, solid or as a SCF. The curves represent the temperatures and pressures where two phases coexist in equilibrium at the triple point, the three phases co-exist. The gas-liquid coexistence curve is known as the boiling curve. If we move upwards along the boiling curve, increasing both temperature and pressure, then the liquid becomes less dense due to thermal expansion and the gas becomes more dense as the pressure rises.
Eventually, the densities of the two phases converge and become identical, the distinction between gas and liquid disappears, and the boiling curve comes to an end at the critical point. The critical point for carbon dioxide occurs at a pressure of Molecules on the surface of a liquid are held to the liquid by the intermolecular attractions from the molecules below and beside them. It arises because atoms on the surface are missing bonds. Energy is released when bonds are formed, so the most stable low energy configuration has the fewest missing bonds.
Surface tension therefore tries to minimize the surface area, resulting in liquids forming spherical droplets and allowing insects to walk on the surface without sinking. Capillary action is a physical effect caused by the interactions of a liquid with the walls of a thin tube. The capillary effect is a function of the ability of the liquid to wet a particular material. The liquid for which this effect is most commonly seen is water, because water is capable of strong surface interactions and because water is ubiquitous.
Water climbs up a thin glass tube because of the strong hydrogen-bonding interactions between the water and the oxygens and terminal hydrogens at the surface of the glass SiO 2 ; surface oxygens are typically bonded to hydrogen. The energetic gain from the new intermolecular interactions must be balanced against gravity, which attempts to pull the liquid back down. Therefore, the narrower the tube, the higher the liquid will climb, because a narrow column of liquid weighs less than a thick one.
Viscosity is an internal property of a fluid that offers resistance to flow.