Exchange current density units for liquid

The exchange current density is the current in the absence of net electrolysis and at zero overpotential. Exchange current densities reflect intrinsic rates of electron transfer between an analyte and the electrode. Owing to this difference, mercury is preferred electrode material at reducing cathodic potentials in aqueous solution.

The net current density is the difference between the cathodic and anodic current density. Such rates provide insights into the structure and bonding in the analyte and the electrode. Of course, factors that change the composition of the electrode, including passivating oxides and adsorbed species on the surface, also influences the electron transfer. This ongoing exchange current density units for liquid in both directions is called the exchange current density. The Tafel equation describes the dependence of current for an electrolytic process to overpotential.
.jpg)
Such rates provide insights into the structure and bonding in the analyte and the electrode. The cathodic current is balanced by the anodic current. The exchange current can be thought of as a background current to which the net current exchange current density units for liquid at various overpotentials is normalized. Owing to this difference, mercury is preferred electrode material at reducing cathodic potentials in aqueous solution.

From Wikipedia, the free encyclopedia. Languages Italiano Edit links. For the concentration dependence of the exchange current density, the following expression is given for a one-electron reaction: Such rates provide insights into the structure and bonding in the analyte and the electrode.
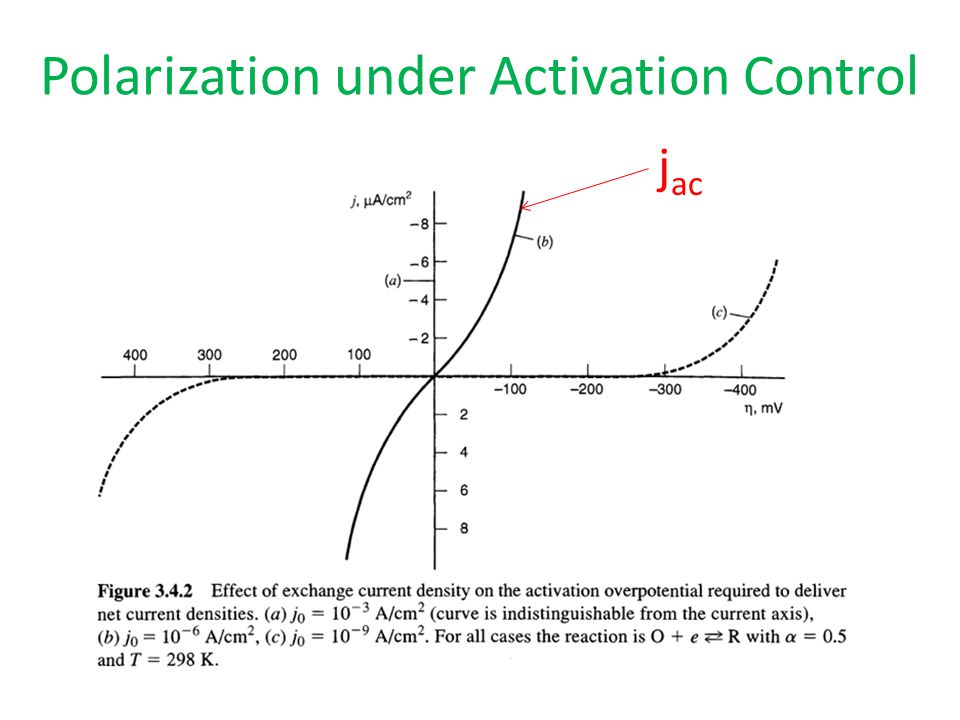
Of course, factors that change the composition of the electrode, including passivating oxides and adsorbed species on the surface, also influences the electron transfer. The nature of the electroactive species the analyte in the solution also critically affects the exchange current densities, both the reduced and oxidized form. Owing to this difference, mercury is preferred electrode material at reducing cathodic potentials in aqueous solution. Exchange current density units for liquid from " https:

Owing to this difference, mercury is preferred electrode material at reducing cathodic potentials in aqueous solution. The exchange current exchange current density units for liquid is the current in the absence of net electrolysis and at zero overpotential. Of course, factors that change the composition of the electrode, including passivating oxides and adsorbed species on the surface, also influences the electron transfer. The Tafel equation describes the dependence of current for an electrolytic process to overpotential.