Diffusion of Nanoparticles in Gases and Liquids
5 stars based on
80 reviews
Diffusion is a process leading to equalization of substance concentrations in a system or establishing in a system an equilibrium concentration distribution that results from random migration of the system's elements. Three types of diffusion are distinguished, viz. Molecular diffusion occurs in gases, liquids, and solids; both diffusion of molecules of extraneous substances impurities and self-diffusion are observed. Molecular diffusion occurs as a result of thermal motion of the molecules.
It proceeds at a maximum rate in gases, at a lower rate in liquids, and at a still lower rate in solids—these differences being accounted for by the nature of thermal motion in these media. In a gaseous phase, molecules possess a certain mean velocity depending on the temperature, but their motion is chaotic and in colliding, they change the direction of this motion. However, on the whole, the molecules of the substance migrate at a velocity much lower than the mean velocity of the molecular free motion.
The higher the pressure, the denser is the molecule packing, the less is the free-path length, and molecular diffusion in gases and liquids are both called slower is the diffusion.
The same occurs as molecule mass and size increase. Conversely, elevation of temperature molecular diffusion in gases and liquids are both called an increase in the free-path length, a decrease in the number of collisions, and growth of free-motion velocity. These factors all lead to a speed-up of diffusion. In liquids, molecular diffusion occurs by jumps of the molecules from one position to another; this arises when the energy of the molecule is high enough to rupture the bonds with the neighboring molecules allowing the molecule to move.
On average, the jump does not exceed an intermolecular spacing, and since in a liquid this is much less than in a gas, the diffusion is substantially lower. Since a liquid is virtually incompressible, the diffusion rate is independent of pressure. Elevation of temperature increases intermolecular spacings and the velocity of vibrations and jumps of molecules, which enhances diffusion. Gases contained in solids diffuse as ions or atoms migrating through interstitials of the crystal lattice, and the same is observed for atoms and ions with a radius much smaller than that of the ion or atom of the base substance constituting the solid.
Diffusion of solid impurities occurs by interchange of sites of atoms and vacancies unoccupied sites of crystal latticeby migration of atoms through interstitials, by a simultaneous cyclic migration of several atoms, by a direct interchange of sites of two neighboring atoms, etc. Each displacement requires imparting to a particle a definite amount of energy activation energy.
Therefore, diffusion is extremely sensitive to temperature elevation, which manifests itself in its exponential dependence on temperature. Nevertheless, even at high temperature, diffusion in solids is much slower than in liquids. So far, the above discussion has focused on the so-called pure concentration diffusion proceeding under the effect of concentration gradient or chemical potential in a medium unaffected by external factors.
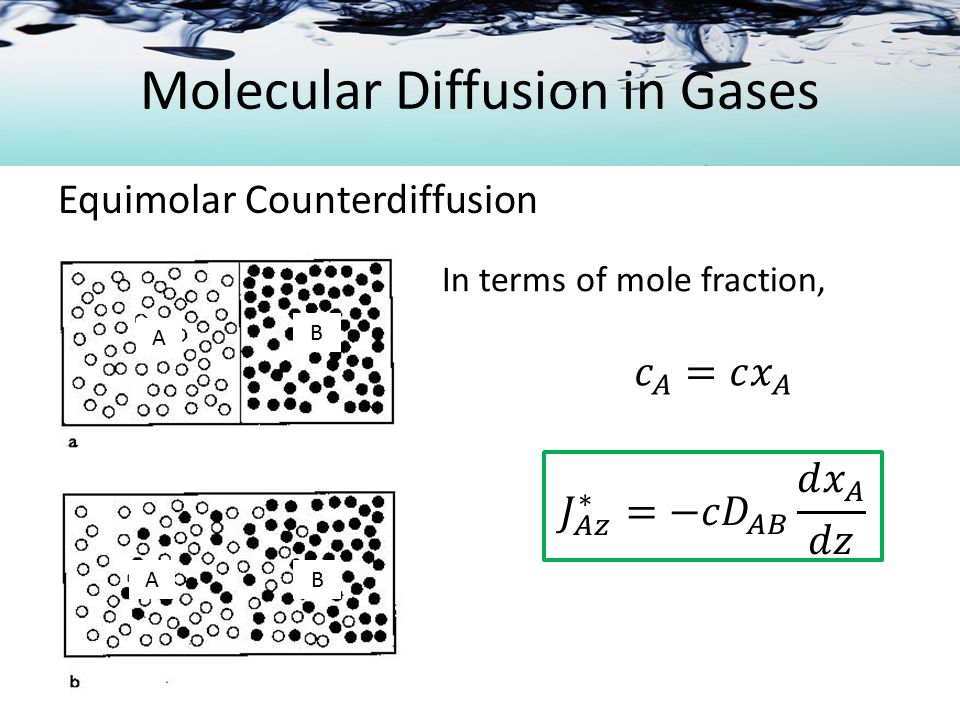
However, it is known that temperature gradient gives rise to a thermal diffusion, pressure gradient to pressure diffusion, an electrical field to electrical diffusion of charged particles, and so on. These diffusion types are beyond the scope of this discussion. In a binary system, the flow of one component must be balanced by the counterflow of the other component. In order to describe a unidirectional diffusion of A molecules in a multicomponent mixture of ideal gases, the Stefan-Maxwell equation.
If fine particles no more than a few microns in size are placed in a stationary gas or liquid at rest, they randomly migrate in the bulk, the motion not decaying and being independent of the medium chemical properties. This phenomenon has come molecular diffusion in gases and liquids are both called be known as Brownian motion.
It is brought about by the absence of a strict compensation of momentum in the molecule-particle collisions, i. The suspended particles migrate independent of each other along intricate zigzag trajectories, changing molecular diffusion in gases and liquids are both called up to 10 14 times a second.
According to the general principles of statistical mechanics, the mean square value of displacement projection of a particle on an arbitrarily-chosen axis is proportional to the observation time t. For spherical particles of radius r, D B is determined by the Stokes-Einstein equation:. Equation 1 has been obtained under the assumption that particle displacements in any direction are equiprobable and, consequently, the mean value of the products of particle displacement in nonoverlapping time intervals t 1 and t 2 is zero: It holds true if we neglect the particle inertia, which is allowed for high enough t's.
If gas or liquid contains in its volume not a single particle but a great number of them, then, by virtue of the statistical character of the process, ultimately the particles appear to be uniformly distributed throughout the volume. In turbulent flows or in artificially-stirred media e. However, under these conditions, mass transfer may also be proportional to the concentration gradient. This fact is indicative of turbulent eddy diffusion. In a turbulent flow, eddies continuously form, break up, disappear and appear once again.
Large-scale eddies may include small-scale ones. The element of substance transferred is not a single molecule of the substance but some quantity of it that depends on the eddy size. The intensity of a turbulent transfer is considerably higher than that resulting from molecular motions.
Treating a nonstationary diffusion of the substance from a point source point 0 yields a simple relation. It has been proven that D E depends on the time of motion for the particle volume considered.
The total mass transfer e. It is assumed in calculations that both processes are additive, i. A substance in electrolyte solutions, particularly dilute ones, exists in the form of ions cations and anions.
The theory of salt diffusion in dilute aqueous solutions is quite well-established, and the diffusion coefficient under an inifinite dilution is determined by the Nernst-Heckell equation. With increasing solution concentration, first decreases and then grows. Molecular diffusion in gases and liquids are both called mixed electrolyte systems with a light cation, e.
In these systems, a unidirectional diffusion proceeds due to a joint action of electrical and concentration gradients. In a multiion system, it is important to allow for ion interaction when several ionic conductions molecular diffusion in gases and liquids are both called differ from other ionic conductions, e. There exist three mechanisms, viz. These can all come into action simultaneously in the same system. Molecular diffusion is predominant in solids with large pores, whose size is much more than the free-path of the diffusing gas molecules.
In this case, diffusion can be described as presented above. Knudsen diffusion occurs in gas-filled solids with small pores, or under low pressure when the mean free-path of molecules is more than the pore size and the molecules collide with the walls more often than between themselves.
Molecule reflection from the walls is normally diffuse, i. The roles Knudsen and molecular diffusions perform are commensurable within a certain range of pore sizes and gas pressures.
Surface diffusion is observed during adsorption of a diffusing substance by a solid. Since the equilibrium surface gas concentration increases with an increase in partial pressure of the adsorbed species, a surface concentration gradient of a diffusing substance appears in the surface layer of a pore.
Under certain conditions, this may enhance the total flow of a diffusing component. For molecular diffusion in porous solids, use is made of the effective diffusion coefficient, arbitrarily related to the concentration gradient of a substance diffusing normal to the external surface of the body. In order to calculate the flux density due to the Knudsen diffusion, it is also advisable to use some effective coefficient.
In a transition region in which both molecular and Knudsen diffusions are essential, an overall effective diffusion coefficient is recommended. The above refers only to a single-component diffusion.
Multicomponent diffusion in molecular diffusion in gases and liquids are both called solids has been inadequately investigated and data of any reliability for its calculation are not available. In considering the role of surface diffusion, it is generally assumed that the mass flux is proportional to the concentration gradient, and the absorbed layer of this sustance is extremely thin and does not change the pore cross section.
Under these assumptions, the mass flux is:. Assuming that an adsorbed layer and a gaseous phase in pores are at equilibrium and that the adsorption isotherm is linear, i. This equation describes the diffusion flux in a porous solid when all the three diffusion mechanisms come into action simultaneously. Diffusion in Electrolyte Solutions.
Diffusion in porous bodies.